This article is a follow-up on the bachelor’s thesis by De Meester (2025, 30–32) researching birch bark extracts, such as betulin and pyrolysis distillates, as a potential sustainable alternative for current commercial wood preservatives. The bachelor’s thesis is part of the KOIVUA project at LAB University of Applied Sciences (LAB University of Applied Sciences 2024). The study showed hydrophobic interactions for all treatment solutions and concluded that both the betulin solution and pyrolysis distillates have the potential to be used as a wood preservative. This is supported by betulin being described in literature as having anti-inflammatory, anti-itching, antibacterial, and anti-mycotic effects. Besides, betulin has a unique capability to stabilize water-in-oil emulsions, making it possible to create emulsifier-free creams using solely oil, water, and betulin. (Holmbom 2011, 204.) Regarding the pyrolysis distillates, the findings from De Meester (2025) are in accordance with a study conducted by Barbero-López et al. (2019). The study tested the growth of three brown-rots against two slow pyrolysis distillates from birch bark at different concentrations. At a concentration of 1% one birch bark distillate showed a 100% inhibition of all three tested brown-rot fungi. The study concluded that slow pyrolysis distillates from birch bark show antifungal activity against wood-decaying fungi. (Barbero-López et al. 2019, 604–610.)
Even though clear and promising results were found, De Meester’s study (2025) emphasized that further refinement of both solutions is required. One of the main challenges in the study was the movement of the spruce samples due to not being placed in a fixed position, and a minimal growth of the fungus Serpula Lacrymans (SL). Water added to the petri dishes caused the spruce samples to float and easily move out of place. Because the fungi were inoculated between the samples, the displacement of these samples has a direct impact on any possible growth of the fungi. (De Meester 2025, 23–27.)
Experimental set-up, fungal growth, and problem areas
This article aims to demonstrate a possible solution to the movement of the spruce samples by comparing an alternative experimental set-up in which the spruce samples are placed on top of the fungi. As can be seen on image 1, in the experimental set-up by De Meester (2025, 24–25) the spruce showed a visually clear growth for Fomitopsis pinicola (FP) on the left and no growth for the pyrolysis distillates inoculated with Serpula Lacrymans (SL) on the right after a 6-month period. In the study, small squares of fungi, cultivated on Malt Extract Agar (MEA), were placed in between the samples. The samples were placed on the petri dish causing them to move and lose contact with the fungi, especially when water accumulated at the bottom of the petri dish. Even though the samples were placed back in place, this could have slowed or even stopped initial fungal growth. Besides, the amount of fungus used to inoculate was relatively small, resulting in slow fungal growth. (De Meester 2025, 17–27.)
Solution to improve the efficiency of the experimental set-up
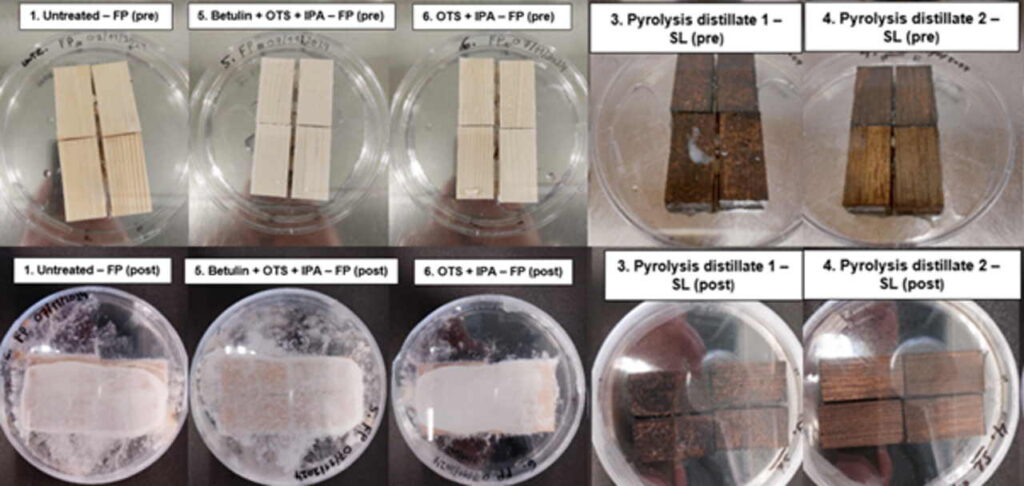
Image 2 shows a pre- and post-test comparison of spruce samples with the same treatments as described above inoculated with the same fungi FP on the left and SL on the right over a 6-month period. Regarding the untreated, betulin and solvent control the growth of FP is much stronger when the samples are placed on top of the fungus. Another interesting appearance is that the growth of SL continued, though away from the samples treated with the pyrolysis distillate. No growth can be found near or on top of the samples. The distance of the growth is slightly further away from the sample treated with pyrolysis distillate 1 (labelled 3/10) compared to the sample treated with pyrolysis distillate 2 (labelled 4/10).

Though, the difference between both pyrolysis distillates is minimal, and more research is required to further analyze both treatments. Besides, the samples in both petri dishes never moved over the entire experimental phase, which was a success compared to the previous study by De Meester (2025, 30–32). These initial results show that placing treated spruce samples on top of a pre-grown fungus could be a more effective way to test the antifungal qualities of birch bark extracts.
Authors
Dieter De Meester studies Sustainable Solutions Engineering at LAB University of Applied Sciences.
Mervi Pulkkinen works as a Laboratory Coordinator in the teaching and analysis laboratories of the Faculty of Technology at LAB University of Applied Sciences. In the KOIVUA project, she works as a specialist.
References
Barbero-López, A., Chibily, S., Tomppo, L., Salami, A., Ancin-Murguzur, F.J., Venäläinen, M., Lappalainen, R. & Haapala, A. 2019. Pyrolysis distillates from tree bark and fibre hemp inhibit the growth of wood-decaying fungi. Industrial Crops and Products, Vol. 129, 604–610. Cited 21 Aug 2025. Available at https://doi.org/10.1016/j.indcrop.2018.12.049
De Meester, D. 2025. The anti-decay properties of birch bark extracts. Bachelor’s thesis. LAB University of Applied Sciences, Sustainable Solutions Engineering. Lahti. Cited 17 Jun 2025. Available at https://urn.fi/URN:NBN:fi:amk-2025061623071
Holmbom, B. 2011. Extraction and utilization of non-structural wood and bark components. Biorefining of Forest Resources, 178–224. Espoo: Paperi ja Puu Oy.
LAB University of Applied Sciences. 2024. Koivun kuoren biojalostuksen mahdollisuudet (KOIVUA-hanke). Cited 17 Jun 2025. Available at https://lab.fi/fi/projekti/koivun-kuoren-biojalostuksen-mahdollisuudet




